zn electron config|electron configuration for every element : Manila 101K views 3 years ago. To write the configuration for the Zinc and the Zinc ion, first we need to write the electron configuration for just Zinc (Zn). We first need to find the number of. Zgarnij bonus bez depozytu od Bruce Bet jeszcze dziś! Zarejestruj się w Bruce Bet, korzystając z naszego odnośnika jeszcze dziś i zgarnij wspaniały bonus bez depozytu na rozpoczęcie swojej przygody z tym kasynem. Nigdzie nie znajdziesz lepszej oferty! I nie zapomnij przejrzeć także innych promocji dostępnych w Bruce Bet, bo bonus bez .
zn electron config,Mar 23, 2023 Click on above elements (in Periodic table) to see their information or Visit . 101K views 3 years ago. To write the configuration for the Zinc and the Zinc ion, first we need to write the electron configuration for just Zinc (Zn). We first need to find the number of.Zinc, electron configuration. Periodic table » Zinc » Electron configuration. Zinc. Full electron configuration of zinc: 1s2 2s2 2p6 3s2 3p6 3d10 4s2. copper ← zinc → gallium. .
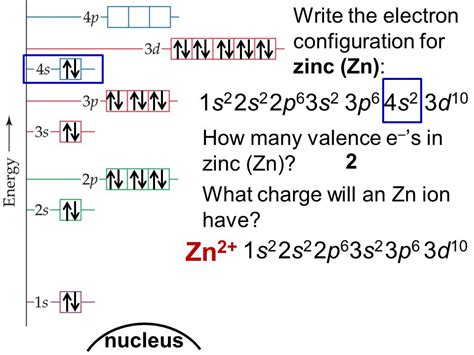
What is the Electron Configuration of Zinc. [Ar] 3d10 4s2 is the electron configuration of Zinc. How Many Valence Electrons does .Electron configuration. The arrangements of electrons above the last (closed shell) noble gas. Melting point. The temperature at which the solid–liquid phase change occurs. .zn electron config electron configuration for every elementElectronic configuration of the Zinc atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10Electronic configuration of the Zinc atom in ascending order . Explanation: For Zn we got Z = 30 ..and thus.. 1s22s22s63s23s63d104s2. .and thus we have distributed 30 electrons as required... Answer link. By taking the .
Peb 1, 2021 3.1: Electron Configurations. Page ID. Skills to Develop. Derive the predicted ground . The atomic number of Zinc (Zn) is 30. Therefore, the electronic configuration of Zinc can be represented as: 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Here, the first shell contains 2 electrons (1s²), the second shell contains 8 electrons (2s² 2p⁶), the third shell contains 18 electrons (3s² 3p⁶), and the fourth shell contains 2 electrons in the s .
Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.The chemical symbol for Zinc is Zn. Electron Configuration and Oxidation States of Zinc. Electron configuration of Zinc is [Ar] 3d10 4s2. Possible oxidation states are +2. Electron Configuration. The periodic . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and .

This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.
How can you find the electron configuration of Zn? Chemistry Electron Configuration Electron Configuration. 1 Answer anor277 Nov 22, 2017 By taking the atomic number of #Zn#, and use of the old #"aufbau principle."# Explanation: For #Zn# we .electron configuration for every elementThe electron configuration and orbital diagram for carbon are: Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule. These three electrons have unpaired spins. . (Zn, Cd, Hg, as well as Cu, Ag, and Au in Figure 6.29) are not technically transition . The electron configuration of a neutral zinc atom is 1s22s22p63s23p63d104s2. The Zn2+ ion has lost two electrons, which leaves it with 30 protons and 28 electrons. The electron configuration of Zn2+ is 1s22s22p63s23p63d10. Zinc is a d-block element, also known as a transition element. For the d-block elements, .Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom.

Explanation: The atom is electrically neutral. Therefore, the number of protons is equal to the number of electrons. 65 30Zn. The zinc atom has 30 protons ⇒ 30 electrons. 1s2,2s2,2p6,3s2,3p6,4s2,3d10. Answer link. 1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10 The atom is electrically neutral.
In general, the electronic configuration of these elements is (n-1) d 1-10 ns 1-2. Here, (n–1) stands for the inner d orbitals which may have one to ten electrons, and the outermost n s orbital may have one or two electrons. Electronic configuration of Zinc Zn: The electronic configuration of Zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Represented in the periodic table as Zn, zinc is a transition metal, grouped with cadmium and mercury. With the middling atomic number 30, it has five stable isotopes of .
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
zn electron configWrite the electron configurations of these cations. Solution. First, write the electron configuration for the neutral atoms: Zn: [Ar]3 d10 4 s2. Cr: [Ar]3 d5 4 s1. Next, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital first and then from the d orbital.
zn electron config|electron configuration for every element
PH0 · what is the electron configuration for zinc
PH1 · lowest energy configuration of zn
PH2 · electron configuration worksheet
PH3 · electron configuration for every element
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · complete electron configuration for gold
PH7 · all element configuration for zn
PH8 · Iba pa